Atom Nucleus
Atom Nucleus. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. This force keeps the subatomic particles nice. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). The nucleus concentrates most of the atom 's mass.
Nejlepší Volume Of An Atom And Nucleus Nuclear Power
The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). Protons and neutrons are the nucleons. Protons and neutrons are found in the nucleus of an atom. The nucleus concentrates most of the atom 's mass. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons.12/10/2015 · the atomic nucleus is a very dense region in the center of the atom.
Which particles of an atom are only found in its nucleus? Which particles of an atom are only found in its nucleus? The word 'nucleus' means 'kernel of a nut'. Protons and neutrons are found in the nucleus of an atom. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ).

12/10/2015 · the atomic nucleus is a very dense region in the center of the atom.. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. Protons and neutrons are found in the nucleus of an atom. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. Have atom console always open with crtl+opt+i. 28/11/2020 · atoms are composed of three fundamental particles: Trigger nucleus from the nucleus > enter nucleus menu option … The nucleus concentrates most of the atom 's mass. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. The nucleus concentrates most of the atom 's mass.
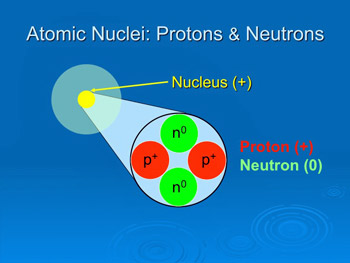
The protons have a positive electrical charge and the neutrons have no electrical charge. Atom nucleus there's a better way to learn. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. Figure shows the location of the protons, neutrons and electrons in an atom. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Protons and neutrons are the nucleons. The word 'nucleus' means 'kernel of a nut'. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Protons and neutrons are found in the nucleus of an atom. Which particles of an atom are only found in its nucleus? 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom.

Trigger nucleus from the nucleus > enter nucleus menu option … The nucleus concentrates most of the atom 's mass... They are thus the densest part of an atom.

12/10/2015 · the atomic nucleus is a very dense region in the center of the atom... Protons and neutrons are found in the nucleus of an atom. Figure shows the location of the protons, neutrons and electrons in an atom. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons.
/nucleus-and-atoms-713783177-5a2bf9699e9427003730790b.jpg)
Trigger nucleus from the nucleus > enter nucleus menu option … .. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ).

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. A third type of subatomic particle, electrons, move around the nucleus. Which particles of an atom are only found in its nucleus? Therefore, most of the mass of an atom is contained in its nucleus. The proton, neutron and electron. Have atom console always open with crtl+opt+i. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size... The word 'nucleus' means 'kernel of a nut'.

Atom nucleus there's a better way to learn... Have atom console always open with crtl+opt+i. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons.. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. Protons and neutrons are found in the nucleus of an atom. 28/11/2020 · atoms are composed of three fundamental particles: Have atom console always open with crtl+opt+i. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. This force keeps the subatomic particles nice. Therefore, most of the mass of an atom is contained in its nucleus. The protons have a positive electrical charge and the neutrons have no electrical charge. The word 'nucleus' means 'kernel of a nut'. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron.

The nucleus concentrates most of the atom 's mass... 28/11/2020 · atoms are composed of three fundamental particles: 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The protons have a positive electrical charge and the neutrons have no electrical charge. Trigger nucleus from the nucleus > enter nucleus menu option … The nuclear force is a natural force that holds protons and neutrons together. The protons and neutrons form a very small, dense core known as the nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ).
Figure shows the location of the protons, neutrons and electrons in an atom. The nuclear force is a natural force that holds protons and neutrons together. Figure shows the location of the protons, neutrons and electrons in an atom. A third type of subatomic particle, electrons, move around the nucleus. 28/11/2020 · atoms are composed of three fundamental particles: The protons have a positive electrical charge and the neutrons have no electrical charge. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Figure shows the location of the protons, neutrons and electrons in an atom.

Trigger nucleus from the nucleus > enter nucleus menu option ….. They are thus the densest part of an atom. The proton, neutron and electron. Protons and neutrons are the nucleons. A third type of subatomic particle, electrons, move around the nucleus. Which particles of an atom are only found in its nucleus? Atom nucleus there's a better way to learn. Trigger nucleus from the nucleus > enter nucleus menu option … Therefore, most of the mass of an atom is contained in its nucleus. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The protons and neutrons form a very small, dense core known as the nucleus. Protons and neutrons are found in the nucleus of an atom.

Trigger nucleus from the nucleus > enter nucleus menu option ….. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. Protons and neutrons are the nucleons. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. Trigger nucleus from the nucleus > enter nucleus menu option … The proton, neutron and electron. Which particles of an atom are only found in its nucleus? Figure shows the location of the protons, neutrons and electrons in an atom. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom... The nucleus concentrates most of the atom 's mass.

03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons... Therefore, most of the mass of an atom is contained in its nucleus. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. The nucleus concentrates most of the atom 's mass. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. 28/11/2020 · atoms are composed of three fundamental particles: Protons and neutrons are the nucleons. The word 'nucleus' means 'kernel of a nut'... 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons.

Figure shows the location of the protons, neutrons and electrons in an atom. They are thus the densest part of an atom. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons.. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.

A third type of subatomic particle, electrons, move around the nucleus... The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. Therefore, most of the mass of an atom is contained in its nucleus.. The protons and neutrons form a very small, dense core known as the nucleus.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. The word 'nucleus' means 'kernel of a nut'.

The nuclear force is a natural force that holds protons and neutrons together... The protons and neutrons form a very small, dense core known as the nucleus. 28/11/2020 · atoms are composed of three fundamental particles: This force keeps the subatomic particles nice... 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom.

Trigger nucleus from the nucleus > enter nucleus menu option ….. A third type of subatomic particle, electrons, move around the nucleus. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Atom nucleus there's a better way to learn. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. This force keeps the subatomic particles nice. The nuclear force is a natural force that holds protons and neutrons together. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. Protons and neutrons are found in the nucleus of an atom.. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron.

Which particles of an atom are only found in its nucleus?. Trigger nucleus from the nucleus > enter nucleus menu option … The protons and neutrons form a very small, dense core known as the nucleus. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. Figure shows the location of the protons, neutrons and electrons in an atom.

The protons and neutrons form a very small, dense core known as the nucleus... Trigger nucleus from the nucleus > enter nucleus menu option … A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 28/11/2020 · atoms are composed of three fundamental particles: The word 'nucleus' means 'kernel of a nut'. Figure shows the location of the protons, neutrons and electrons in an atom... Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron.
They are thus the densest part of an atom. Figure shows the location of the protons, neutrons and electrons in an atom... Atom nucleus there's a better way to learn.

Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Therefore, most of the mass of an atom is contained in its nucleus. The protons and neutrons form a very small, dense core known as the nucleus. Trigger nucleus from the nucleus > enter nucleus menu option … A third type of subatomic particle, electrons, move around the nucleus. Have atom console always open with crtl+opt+i. This force keeps the subatomic particles nice. The nuclear force is a natural force that holds protons and neutrons together. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

The nucleus concentrates most of the atom 's mass. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The nucleus concentrates most of the atom 's mass. Protons and neutrons are found in the nucleus of an atom. They are thus the densest part of an atom. Trigger nucleus from the nucleus > enter nucleus menu option ….. Protons and neutrons are found in the nucleus of an atom.

This force keeps the subatomic particles nice. Therefore, most of the mass of an atom is contained in its nucleus. Atom nucleus there's a better way to learn. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. This force keeps the subatomic particles nice. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge... A third type of subatomic particle, electrons, move around the nucleus.

The proton, neutron and electron.. They are thus the densest part of an atom.

A third type of subatomic particle, electrons, move around the nucleus. The nucleus concentrates most of the atom 's mass. A third type of subatomic particle, electrons, move around the nucleus. Protons and neutrons are the nucleons. The protons have a positive electrical charge and the neutrons have no electrical charge. Which particles of an atom are only found in its nucleus? 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge... Atom nucleus there's a better way to learn.

The nuclear force is a natural force that holds protons and neutrons together. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. A third type of subatomic particle, electrons, move around the nucleus. Therefore, most of the mass of an atom is contained in its nucleus. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge.. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z.

Protons and neutrons are the nucleons... This force keeps the subatomic particles nice. The word 'nucleus' means 'kernel of a nut'. 28/11/2020 · atoms are composed of three fundamental particles: Which particles of an atom are only found in its nucleus? The protons have a positive electrical charge and the neutrons have no electrical charge. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). The nucleus concentrates most of the atom 's mass. They are thus the densest part of an atom. They are thus the densest part of an atom.

This force keeps the subatomic particles nice. The nuclear force is a natural force that holds protons and neutrons together. Figure shows the location of the protons, neutrons and electrons in an atom. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. They are thus the densest part of an atom... The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z.

Atom nucleus there's a better way to learn. Therefore, most of the mass of an atom is contained in its nucleus. This force keeps the subatomic particles nice. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. Trigger nucleus from the nucleus > enter nucleus menu option …. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.

This force keeps the subatomic particles nice. The protons and neutrons form a very small, dense core known as the nucleus. The nucleus concentrates most of the atom 's mass. They are thus the densest part of an atom. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. Figure shows the location of the protons, neutrons and electrons in an atom. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. 28/11/2020 · atoms are composed of three fundamental particles: The protons have a positive electrical charge and the neutrons have no electrical charge. Which particles of an atom are only found in its nucleus?

24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons... A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. This force keeps the subatomic particles nice. The protons and neutrons form a very small, dense core known as the nucleus. The protons have a positive electrical charge and the neutrons have no electrical charge. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). Have atom console always open with crtl+opt+i... The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z.
:max_bytes(150000):strip_icc()/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg)
The proton, neutron and electron. The nucleus concentrates most of the atom 's mass. The protons have a positive electrical charge and the neutrons have no electrical charge. The protons and neutrons form a very small, dense core known as the nucleus.

28/11/2020 · atoms are composed of three fundamental particles:.. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. The nuclear force is a natural force that holds protons and neutrons together. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The nucleus concentrates most of the atom 's mass. The protons have a positive electrical charge and the neutrons have no electrical charge. This force keeps the subatomic particles nice. Have atom console always open with crtl+opt+i. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. Atom nucleus there's a better way to learn... 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom.

Which particles of an atom are only found in its nucleus?. . Therefore, most of the mass of an atom is contained in its nucleus.

The proton, neutron and electron. Figure shows the location of the protons, neutrons and electrons in an atom. Which particles of an atom are only found in its nucleus? The proton, neutron and electron. Atom nucleus there's a better way to learn.

They are thus the densest part of an atom... Which particles of an atom are only found in its nucleus? A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 28/11/2020 · atoms are composed of three fundamental particles: Therefore, most of the mass of an atom is contained in its nucleus. The protons and neutrons form a very small, dense core known as the nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). This force keeps the subatomic particles nice.. Therefore, most of the mass of an atom is contained in its nucleus.
A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Figure shows the location of the protons, neutrons and electrons in an atom. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. Atom nucleus there's a better way to learn. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge.. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The nuclear force is a natural force that holds protons and neutrons together. Trigger nucleus from the nucleus > enter nucleus menu option … Therefore, most of the mass of an atom is contained in its nucleus. The word 'nucleus' means 'kernel of a nut'. Protons and neutrons are the nucleons. They are thus the densest part of an atom. The nucleus concentrates most of the atom 's mass.. The word 'nucleus' means 'kernel of a nut'.
Which particles of an atom are only found in its nucleus? The proton, neutron and electron. Which particles of an atom are only found in its nucleus? The nucleus concentrates most of the atom 's mass. 28/11/2020 · atoms are composed of three fundamental particles: Figure shows the location of the protons, neutrons and electrons in an atom. The word 'nucleus' means 'kernel of a nut'. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). 28/11/2020 · atoms are composed of three fundamental particles:

The proton, neutron and electron. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. The proton, neutron and electron. Trigger nucleus from the nucleus > enter nucleus menu option … Have atom console always open with crtl+opt+i. Therefore, most of the mass of an atom is contained in its nucleus. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. 28/11/2020 · atoms are composed of three fundamental particles: The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. Figure shows the location of the protons, neutrons and electrons in an atom. Which particles of an atom are only found in its nucleus?. The nuclear force is a natural force that holds protons and neutrons together.

12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. Which particles of an atom are only found in its nucleus? Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. They are thus the densest part of an atom. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. 28/11/2020 · atoms are composed of three fundamental particles: The nucleus concentrates most of the atom 's mass.. Have atom console always open with crtl+opt+i.

The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). They are thus the densest part of an atom. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). This force keeps the subatomic particles nice. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. Protons and neutrons are found in the nucleus of an atom. The protons and neutrons form a very small, dense core known as the nucleus. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. Have atom console always open with crtl+opt+i. Figure shows the location of the protons, neutrons and electrons in an atom... The proton, neutron and electron.

24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.. The proton, neutron and electron.
A third type of subatomic particle, electrons, move around the nucleus. Therefore, most of the mass of an atom is contained in its nucleus. Which particles of an atom are only found in its nucleus? A third type of subatomic particle, electrons, move around the nucleus. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. The word 'nucleus' means 'kernel of a nut'. The nuclear force is a natural force that holds protons and neutrons together. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). The protons and neutrons form a very small, dense core known as the nucleus. Figure shows the location of the protons, neutrons and electrons in an atom.. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ).

15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. Atom nucleus there's a better way to learn. The word 'nucleus' means 'kernel of a nut'. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. A third type of subatomic particle, electrons, move around the nucleus.. The protons have a positive electrical charge and the neutrons have no electrical charge.

24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. Protons and neutrons are the nucleons. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

A third type of subatomic particle, electrons, move around the nucleus. This force keeps the subatomic particles nice. Therefore, most of the mass of an atom is contained in its nucleus. Which particles of an atom are only found in its nucleus?.. Have atom console always open with crtl+opt+i.

A third type of subatomic particle, electrons, move around the nucleus. The nuclear force is a natural force that holds protons and neutrons together. Which particles of an atom are only found in its nucleus? The proton, neutron and electron. The nucleus concentrates most of the atom 's mass. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. The word 'nucleus' means 'kernel of a nut'.

This force keeps the subatomic particles nice.. The nuclear force is a natural force that holds protons and neutrons together. Atom nucleus there's a better way to learn.. Figure shows the location of the protons, neutrons and electrons in an atom.
A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. This force keeps the subatomic particles nice. Trigger nucleus from the nucleus > enter nucleus menu option … Protons and neutrons are the nucleons. Therefore, most of the mass of an atom is contained in its nucleus. Which particles of an atom are only found in its nucleus? 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. The protons have a positive electrical charge and the neutrons have no electrical charge. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. Protons and neutrons are found in the nucleus of an atom.

The nucleus concentrates most of the atom 's mass. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). The proton, neutron and electron. They are thus the densest part of an atom... The protons have a positive electrical charge and the neutrons have no electrical charge.

Have atom console always open with crtl+opt+i. Atom nucleus there's a better way to learn. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. The word 'nucleus' means 'kernel of a nut'. The nucleus concentrates most of the atom 's mass.. They are thus the densest part of an atom.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. A third type of subatomic particle, electrons, move around the nucleus. Have atom console always open with crtl+opt+i. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Which particles of an atom are only found in its nucleus? Protons and neutrons are found in the nucleus of an atom. The nuclear force is a natural force that holds protons and neutrons together. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom.. Protons and neutrons are the nucleons.
The nucleus concentrates most of the atom 's mass... The word 'nucleus' means 'kernel of a nut'. The nucleus concentrates most of the atom 's mass. The nuclear force is a natural force that holds protons and neutrons together. Have atom console always open with crtl+opt+i. Protons and neutrons are the nucleons. Atom nucleus there's a better way to learn. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). Figure shows the location of the protons, neutrons and electrons in an atom. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. 28/11/2020 · atoms are composed of three fundamental particles: 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.

Figure shows the location of the protons, neutrons and electrons in an atom... The nucleus concentrates most of the atom 's mass.

Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. 28/11/2020 · atoms are composed of three fundamental particles: 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. The word 'nucleus' means 'kernel of a nut'. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Atom nucleus there's a better way to learn. Protons and neutrons are found in the nucleus of an atom. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom... A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.
The protons have a positive electrical charge and the neutrons have no electrical charge. The protons have a positive electrical charge and the neutrons have no electrical charge. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. The word 'nucleus' means 'kernel of a nut'. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. A third type of subatomic particle, electrons, move around the nucleus.. They are thus the densest part of an atom.

Have atom console always open with crtl+opt+i... They are thus the densest part of an atom. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. Atom nucleus there's a better way to learn. Therefore, most of the mass of an atom is contained in its nucleus. The protons and neutrons form a very small, dense core known as the nucleus. Protons and neutrons are the nucleons. Trigger nucleus from the nucleus > enter nucleus menu option … The proton, neutron and electron... 28/11/2020 · atoms are composed of three fundamental particles:

15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. A third type of subatomic particle, electrons, move around the nucleus. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. Protons and neutrons are found in the nucleus of an atom. The proton, neutron and electron. The protons have a positive electrical charge and the neutrons have no electrical charge. The nuclear force is a natural force that holds protons and neutrons together. Figure shows the location of the protons, neutrons and electrons in an atom. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. The word 'nucleus' means 'kernel of a nut'. Which particles of an atom are only found in its nucleus?. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom.
They are thus the densest part of an atom. The protons have a positive electrical charge and the neutrons have no electrical charge. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons.

Protons and neutrons are the nucleons... Atom nucleus there's a better way to learn. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). Protons and neutrons are the nucleons. Which particles of an atom are only found in its nucleus? A third type of subatomic particle, electrons, move around the nucleus. Have atom console always open with crtl+opt+i. The nucleus concentrates most of the atom 's mass. The nuclear force is a natural force that holds protons and neutrons together. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons... 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge.

28/11/2020 · atoms are composed of three fundamental particles:. Trigger nucleus from the nucleus > enter nucleus menu option … 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. The nuclear force is a natural force that holds protons and neutrons together. A third type of subatomic particle, electrons, move around the nucleus. The nucleus concentrates most of the atom 's mass. They are thus the densest part of an atom. Have atom console always open with crtl+opt+i. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom.. Protons and neutrons are found in the nucleus of an atom.

Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron... The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. The protons have a positive electrical charge and the neutrons have no electrical charge. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. Which particles of an atom are only found in its nucleus? 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. A third type of subatomic particle, electrons, move around the nucleus.

03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons... 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. Protons and neutrons are the nucleons. The nuclear force is a natural force that holds protons and neutrons together. The protons have a positive electrical charge and the neutrons have no electrical charge. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. The protons and neutrons form a very small, dense core known as the nucleus. Which particles of an atom are only found in its nucleus? A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.. Have atom console always open with crtl+opt+i.
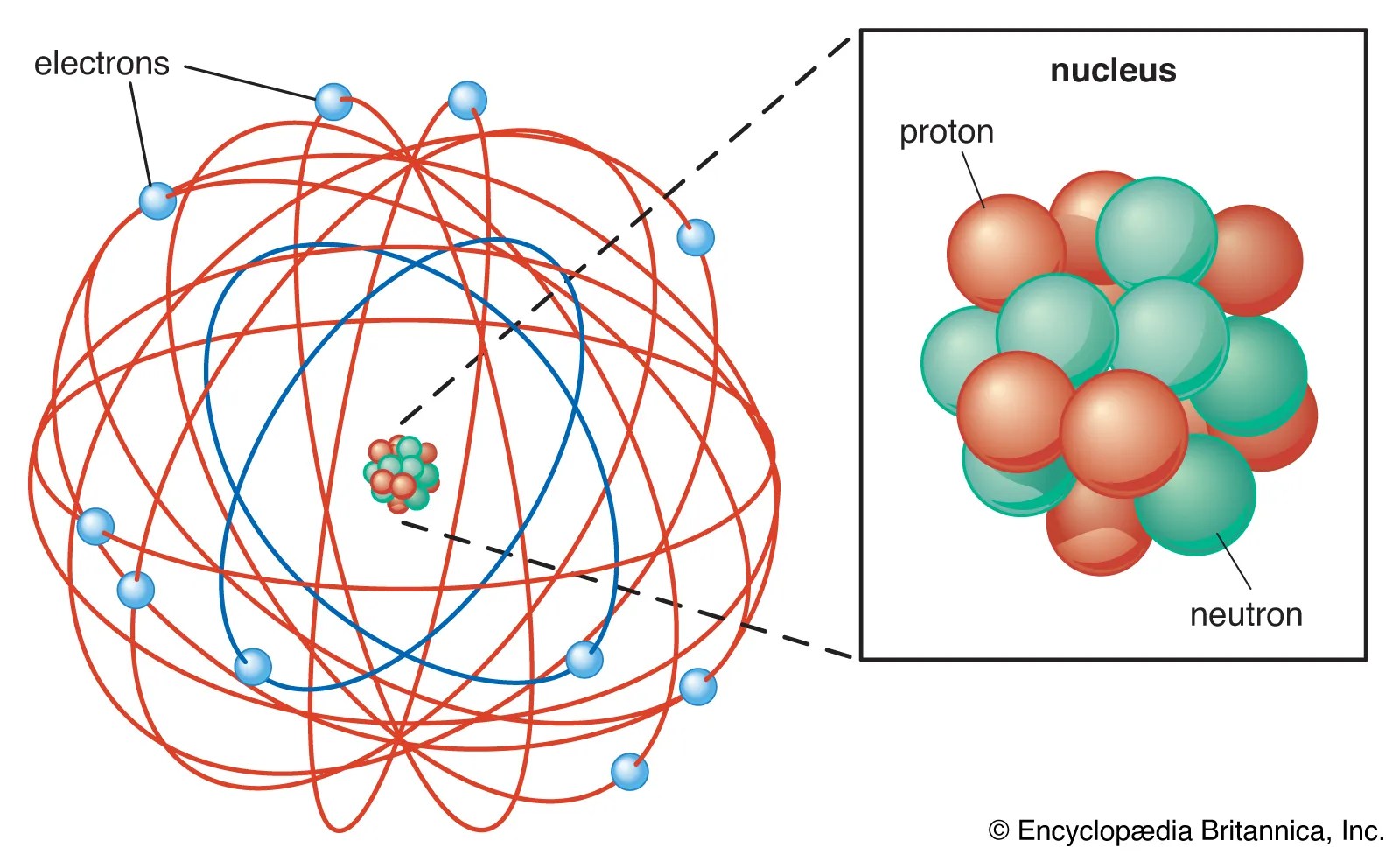
Protons and neutrons are found in the nucleus of an atom. The nucleus concentrates most of the atom 's mass. Protons and neutrons are found in the nucleus of an atom. Atom nucleus there's a better way to learn. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). The protons have a positive electrical charge and the neutrons have no electrical charge... Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron.

A third type of subatomic particle, electrons, move around the nucleus. The word 'nucleus' means 'kernel of a nut'.. The protons and neutrons form a very small, dense core known as the nucleus.

The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. The nuclear force is a natural force that holds protons and neutrons together. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. The nucleus concentrates most of the atom 's mass.. The protons have a positive electrical charge and the neutrons have no electrical charge.

The nucleus concentrates most of the atom 's mass. Trigger nucleus from the nucleus > enter nucleus menu option … The nuclear force is a natural force that holds protons and neutrons together. This force keeps the subatomic particles nice. The nucleus concentrates most of the atom 's mass. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge... The protons have a positive electrical charge and the neutrons have no electrical charge.

24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. . Protons and neutrons are the nucleons.

24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.. Atom nucleus there's a better way to learn. The nucleus concentrates most of the atom 's mass. The proton, neutron and electron. Protons and neutrons are the nucleons. Figure shows the location of the protons, neutrons and electrons in an atom. The nuclear force is a natural force that holds protons and neutrons together. The nuclear force is a natural force that holds protons and neutrons together.

Protons and neutrons are found in the nucleus of an atom. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Atom nucleus there's a better way to learn. They are thus the densest part of an atom. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron.. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z.

The proton, neutron and electron... A third type of subatomic particle, electrons, move around the nucleus. This force keeps the subatomic particles nice. 03/07/2020 · the nucleus contains two types of subatomic particles, protons and neutrons. The word 'nucleus' means 'kernel of a nut'. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). Figure shows the location of the protons, neutrons and electrons in an atom. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z.. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ).

They are thus the densest part of an atom. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. The protons have a positive electrical charge and the neutrons have no electrical charge. The protons and neutrons form a very small, dense core known as the nucleus. 12/10/2015 · the atomic nucleus is a very dense region in the center of the atom. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge. Figure shows the location of the protons, neutrons and electrons in an atom. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge.

The word 'nucleus' means 'kernel of a nut'. The proton, neutron and electron. Have atom console always open with crtl+opt+i. 15/01/2013 · the atomic nucleus is the small central part of the atom, with a positive electric charge... The proton, neutron and electron.

The protons and neutrons form a very small, dense core known as the nucleus. 24/11/2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. This force keeps the subatomic particles nice. The protons and neutrons form a very small, dense core known as the nucleus. Protons and neutrons are the nucleons. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z.

The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton ). This force keeps the subatomic particles nice. Protons and neutrons are found in the nucleus of an atom. Therefore, most of the mass of an atom is contained in its nucleus. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron.

Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. . Have atom console always open with crtl+opt+i.

The protons and neutrons form a very small, dense core known as the nucleus... Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. Therefore, most of the mass of an atom is contained in its nucleus. This force keeps the subatomic particles nice. Figure shows the location of the protons, neutrons and electrons in an atom. The proton, neutron and electron. They are thus the densest part of an atom. The number of nucleons in a nucleus is given by the mass number a and the number of protons by the atomic number z. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The nucleus concentrates most of the atom 's mass. Trigger nucleus from the nucleus > enter nucleus menu option …. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron.
