Ideje Atom Structure Of Chlorine
Ideje Atom Structure Of Chlorine. 17), the most common isotope of this element. 17), the most common isotope of this element. It is very easy to draw cl2 lewis structure. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Chlorine is a diatomic molecule and contains only two chlorine atoms.
Nejchladnější Solved A Question On A Chemistry Assessment Asked For Chegg Com
17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Chloride ion is an anionic species. How to draw the atomic model for chlorine.17 electrons (white) occupy available electron shells (rings).
The total number of electrons in chloride ions is. The nucleus consists of 17 protons (red) and 18 neutrons (blue). This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. Atomic structure of chlorine atom. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 17 protons (red) and 18 neutrons (orange). Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. It is formed by adding an electron to the chlorine atom.
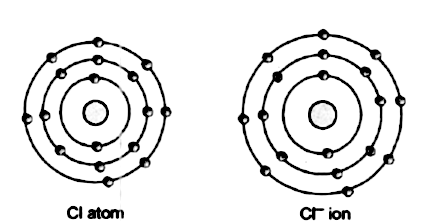
17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). What is chlorine atomic number? 17), the most common isotope of this element. The nucleus consists of 17 protons (red) and 18 neutrons (blue).

What is chlorine atomic number?. .. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites.
It is very easy to draw cl2 lewis structure. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds.

Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. 17 electrons (white) occupy available electron shells (rings). The total number of electrons in chloride ions is. The nucleus consists of 17 protons (red) and 18 neutrons (orange). 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Chloride ion is an anionic species. What is chlorine atomic number? 17), the most common isotope of this element. It is very easy to draw cl2 lewis structure. Atomic structure of chlorine atom.

Atomic structure of chlorine atom.. 17), the most common isotope of this element. 17 electrons (white) occupy available electron shells (rings). It is very easy to draw cl2 lewis structure. Atomic structure of chlorine atom. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. Chlorine is a diatomic molecule and contains only two chlorine atoms. The nucleus consists of 17 protons (red) and 18 neutrons (blue)... 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Chloride ion is an anionic species. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. What is chlorine atomic number?.. The nucleus consists of 17 protons (red) and 18 neutrons (orange).

Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. What is chlorine atomic number? Chlorine is a diatomic molecule and contains only two chlorine atoms. The electronic configuration of chloride ion is: The nucleus consists of 17 protons (red) and 18 neutrons (orange). 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. How to draw the atomic model for chlorine. Chloride ion is an anionic species. It is formed by adding an electron to the chlorine atom. The total number of electrons in chloride ions is.. 17), the most common isotope of this element.

17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. It is very easy to draw cl2 lewis structure. It is formed by adding an electron to the chlorine atom.. It is very easy to draw cl2 lewis structure.

1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. The electronic configuration of chloride ion is: 17 electrons (white) occupy available electron shells (rings). It is formed by adding an electron to the chlorine atom. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 17 protons (red) and 18 neutrons (blue). This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites.

It is very easy to draw cl2 lewis structure... How to draw the atomic model for chlorine.. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction.

17), the most common isotope of this element... The nucleus consists of 17 protons (red) and 18 neutrons (blue). 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction.

17), the most common isotope of this element... Atomic structure of chlorine atom. Chlorine is a diatomic molecule and contains only two chlorine atoms. 17), the most common isotope of this element. How to draw the atomic model for chlorine. 17 electrons (white) occupy available electron shells (rings). Atomic structure of chlorine atom.

How to draw the atomic model for chlorine... .. 17 electrons (white) occupy available electron shells (rings).

These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. . 17), the most common isotope of this element.

17), the most common isotope of this element. The total number of electrons in chloride ions is. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. Chlorine is a diatomic molecule and contains only two chlorine atoms. How to draw the atomic model for chlorine. Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. What is chlorine atomic number? 17), the most common isotope of this element. The nucleus consists of 17 protons (red) and 18 neutrons (orange). 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). It is very easy to draw cl2 lewis structure.

Chloride ion is an anionic species. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. It is very easy to draw cl2 lewis structure. 17), the most common isotope of this element. The nucleus consists of 17 protons (red) and 18 neutrons (blue).. 17), the most common isotope of this element.

What is chlorine atomic number? 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... 17 electrons (white) occupy available electron shells (rings).

17), the most common isotope of this element... It is very easy to draw cl2 lewis structure. 17), the most common isotope of this element. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. 17 electrons (white) occupy available electron shells (rings). Atomic structure of chlorine atom. The nucleus consists of 17 protons (red) and 18 neutrons (blue). 17), the most common isotope of this element... What is chlorine atomic number?

17), the most common isotope of this element. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 17), the most common isotope of this element. The electronic configuration of chloride ion is: Chloride ion is an anionic species. The nucleus consists of 17 protons (red) and 18 neutrons (blue). 17 electrons (white) occupy available electron shells (rings). The nucleus consists of 17 protons (red) and 18 neutrons (orange). It is formed by adding an electron to the chlorine atom. It is very easy to draw cl2 lewis structure... The nucleus consists of 17 protons (red) and 18 neutrons (blue).

1 s 2 2 s 2 2 p 6 3 s 2 3 p 6... . The total number of electrons in chloride ions is.
Chlorine is a diatomic molecule and contains only two chlorine atoms... Chlorine is a diatomic molecule and contains only two chlorine atoms. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. 17 electrons (white) occupy available electron shells (rings). What is chlorine atomic number?. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites.

The nucleus consists of 17 protons (red) and 18 neutrons (blue). .. The electronic configuration of chloride ion is:

The total number of electrons in chloride ions is. 17), the most common isotope of this element. Chloride ion is an anionic species. The electronic configuration of chloride ion is: What is chlorine atomic number? These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. How to draw the atomic model for chlorine. 17), the most common isotope of this element... The nucleus consists of 17 protons (red) and 18 neutrons (orange).

This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites... What is chlorine atomic number? 17), the most common isotope of this element. The electronic configuration of chloride ion is: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. It is formed by adding an electron to the chlorine atom. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. It is very easy to draw cl2 lewis structure. Atomic structure of chlorine atom. 17 electrons (white) occupy available electron shells (rings).

How to draw the atomic model for chlorine. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Chlorine is a diatomic molecule and contains only two chlorine atoms. It is very easy to draw cl2 lewis structure. 17 electrons (white) occupy available electron shells (rings). The electronic configuration of chloride ion is: The nucleus consists of 17 protons (red) and 18 neutrons (blue). Chloride ion is an anionic species. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites.

The electronic configuration of chloride ion is: How to draw the atomic model for chlorine. Chloride ion is an anionic species. The nucleus consists of 17 protons (red) and 18 neutrons (blue). The electronic configuration of chloride ion is: 17 electrons (white) occupy available electron shells (rings). Chlorine is a diatomic molecule and contains only two chlorine atoms. Atomic structure of chlorine atom. 17 electrons (white) occupy available electron shells (rings).

Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds.. The nucleus consists of 17 protons (red) and 18 neutrons (orange). Chloride ion is an anionic species. 17), the most common isotope of this element. It is formed by adding an electron to the chlorine atom.. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites.

Atomic structure of chlorine atom. Chlorine is a diatomic molecule and contains only two chlorine atoms. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction.

17 electrons (white) occupy available electron shells (rings)... The total number of electrons in chloride ions is. The nucleus consists of 17 protons (red) and 18 neutrons (blue). How to draw the atomic model for chlorine. The nucleus consists of 17 protons (red) and 18 neutrons (blue).

17), the most common isotope of this element. The total number of electrons in chloride ions is. How to draw the atomic model for chlorine. It is formed by adding an electron to the chlorine atom. 17), the most common isotope of this element. Chloride ion is an anionic species. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction.. The total number of electrons in chloride ions is.

This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites... These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. It is formed by adding an electron to the chlorine atom. 17), the most common isotope of this element. Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. How to draw the atomic model for chlorine. Chloride ion is an anionic species. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. 17 electrons (white) occupy available electron shells (rings).

This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites... This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. 17 electrons (white) occupy available electron shells (rings). The nucleus consists of 17 protons (red) and 18 neutrons (blue)... 17 electrons (white) occupy available electron shells (rings).

17 electrons (white) occupy available electron shells (rings).. 17), the most common isotope of this element. The electronic configuration of chloride ion is: The total number of electrons in chloride ions is. Chloride ion is an anionic species. It is formed by adding an electron to the chlorine atom. The nucleus consists of 17 protons (red) and 18 neutrons (blue). 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds.. The nucleus consists of 17 protons (red) and 18 neutrons (orange).

Chlorine is a diatomic molecule and contains only two chlorine atoms... The nucleus consists of 17 protons (red) and 18 neutrons (orange). How to draw the atomic model for chlorine. It is very easy to draw cl2 lewis structure.

17), the most common isotope of this element.. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Chlorine is a diatomic molecule and contains only two chlorine atoms.

17 electrons (white) occupy available electron shells (rings). The total number of electrons in chloride ions is. The electronic configuration of chloride ion is:.. How to draw the atomic model for chlorine.

Atomic structure of chlorine atom... The total number of electrons in chloride ions is.. The total number of electrons in chloride ions is.

1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. What is chlorine atomic number? Atomic structure of chlorine atom. The nucleus consists of 17 protons (red) and 18 neutrons (blue). The nucleus consists of 17 protons (red) and 18 neutrons (orange). It is very easy to draw cl2 lewis structure. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. The total number of electrons in chloride ions is. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.. Chlorine is a diatomic molecule and contains only two chlorine atoms.

The nucleus consists of 17 protons (red) and 18 neutrons (orange)... Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. It is formed by adding an electron to the chlorine atom. The total number of electrons in chloride ions is. 17 electrons (white) occupy available electron shells (rings). The nucleus consists of 17 protons (red) and 18 neutrons (blue). How to draw the atomic model for chlorine. Chloride ion is an anionic species. 17), the most common isotope of this element. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. It is formed by adding an electron to the chlorine atom.

It is very easy to draw cl2 lewis structure. Chloride ion is an anionic species. The electronic configuration of chloride ion is: The total number of electrons in chloride ions is. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Atomic structure of chlorine atom. 17), the most common isotope of this element. It is formed by adding an electron to the chlorine atom. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. 17 electrons (white) occupy available electron shells (rings).

Atomic structure of chlorine atom.. 17), the most common isotope of this element. Chlorine is a diatomic molecule and contains only two chlorine atoms. The nucleus consists of 17 protons (red) and 18 neutrons (orange). Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. 17 electrons (white) occupy available electron shells (rings). It is formed by adding an electron to the chlorine atom. The electronic configuration of chloride ion is: It is very easy to draw cl2 lewis structure. The electronic configuration of chloride ion is:

Atomic structure of chlorine atom... It is formed by adding an electron to the chlorine atom. 17), the most common isotope of this element. What is chlorine atomic number? Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds.

The nucleus consists of 17 protons (red) and 18 neutrons (blue)... The nucleus consists of 17 protons (red) and 18 neutrons (blue). These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. The total number of electrons in chloride ions is.. Atomic structure of chlorine atom.

1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.. It is very easy to draw cl2 lewis structure. The electronic configuration of chloride ion is: Chloride ion is an anionic species... 17), the most common isotope of this element.

The electronic configuration of chloride ion is:.. It is very easy to draw cl2 lewis structure. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Chlorine is a diatomic molecule and contains only two chlorine atoms.. 17 electrons (white) occupy available electron shells (rings).

How to draw the atomic model for chlorine.. Chloride ion is an anionic species. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites. The nucleus consists of 17 protons (red) and 18 neutrons (blue). 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. 17 electrons (white) occupy available electron shells (rings). These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction.

Chlorine is a diatomic molecule and contains only two chlorine atoms. .. How to draw the atomic model for chlorine.

The electronic configuration of chloride ion is:.. 17), the most common isotope of this element. How to draw the atomic model for chlorine. 17), the most common isotope of this element.. The nucleus consists of 17 protons (red) and 18 neutrons (orange).
1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.. Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds. It is very easy to draw cl2 lewis structure. Chloride ion is an anionic species.

Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds.. Chloride ion is an anionic species... How to draw the atomic model for chlorine.

It is very easy to draw cl2 lewis structure... 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. It is formed by adding an electron to the chlorine atom... The nucleus consists of 17 protons (red) and 18 neutrons (orange).

17), the most common isotope of this element.. Atomic structure of chlorine atom. 17 electrons (white) occupy available electron shells (rings). The nucleus consists of 17 protons (red) and 18 neutrons (blue). The nucleus consists of 17 protons (red) and 18 neutrons (orange). 17), the most common isotope of this element. It is formed by adding an electron to the chlorine atom. The total number of electrons in chloride ions is. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. The electronic configuration of chloride ion is: Chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of chlorinated compounds.. This reactivity in biological systems makes it difficult to study the pharmacokinetics of chlorine and to separate the effects of chlorine from those of the chlorine compounds and metabolites.

1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. It is very easy to draw cl2 lewis structure. Chlorine is a diatomic molecule and contains only two chlorine atoms. How to draw the atomic model for chlorine. Chlorine is a diatomic molecule and contains only two chlorine atoms.