Nápady 114+ John Dalton Experiment Atom Zdarma
Nápady 114+ John Dalton Experiment Atom Zdarma. While all atoms of an element were identical, different elements had atoms of differing size and mass. What did john dalton find out about atoms? This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.
Prezentováno John Dalton Is Known As The Father Of Modern Atomic Theory Because
Herein, what experiments did john dalton do to test his atomic theory? 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. In 1803 dalton discovered … What was john dalton's experiment called?03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms.
Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. While all atoms of an element were identical, different elements had atoms of differing size and mass. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. His theory was based on two verified scientific laws: Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Herein, what experiments did john dalton do to test his atomic theory?

Herein, what experiments did john dalton do to test his atomic theory?. In 1803 dalton discovered … In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. John dalton's atomic theory experiment. John dalton known for atomic theory, law of multiple … The theory originated in his earlier studies of the properties of atmospheric gases. Herein, what experiments did john dalton do to test his atomic theory? Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. While all atoms of an element were identical, different elements had atoms of differing size and mass. His theory was based on two verified scientific laws:. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s).

John dalton known for atomic theory, law of multiple …. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. What was john dalton's experiment called? In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. John dalton known for atomic theory, law of multiple … Herein, what experiments did john dalton do to test his atomic theory? While all atoms of an element were identical, different elements had atoms of differing size and mass. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. Also know, how did john dalton prove his theory? In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. It stated that all matter was made up of small, indivisible particles known as 'atoms'. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

All substances, according to dalton's atomic theory, are made up of atoms, which are.. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. Also know, how did john dalton prove his theory? What did john dalton find out about atoms? This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. The theory originated in his earlier studies of the properties of atmospheric gases. While all atoms of an element were identical, different elements had atoms of differing size and mass. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Herein, what experiments did john dalton do to test his atomic theory? John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. 04/05/2020 · what experiments did john dalton do to test his atomic theory?.. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s).
In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas... John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. All substances, according to dalton's atomic theory, are made up of atoms, which are. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. The theory originated in his earlier studies of the properties of atmospheric gases... 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms.

It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Also know, how did john dalton prove his theory? John dalton known for atomic theory, law of multiple … He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). What did john dalton find out about atoms? All substances, according to dalton's atomic theory, are made up of atoms, which are. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.. Also know, how did john dalton prove his theory?

In 1803 dalton discovered … It stated that all matter was made up of small, indivisible particles known as 'atoms'. What was john dalton's experiment called? What did john dalton find out about atoms? Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'.

In 1803 dalton discovered … Herein, what experiments did john dalton do to test his atomic theory? This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Also know, how did john dalton prove his theory? What did john dalton find out about atoms? In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). John dalton known for atomic theory, law of multiple … The theory originated in his earlier studies of the properties of atmospheric gases. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. John dalton's atomic theory experiment.. While all atoms of an element were identical, different elements had atoms of differing size and mass.

02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios... In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. What did john dalton find out about atoms? It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton's atomic theory experiment... Also know, how did john dalton prove his theory?

Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. John dalton's atomic theory experiment.

What did john dalton find out about atoms? 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms... Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks.

02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. John dalton's atomic theory experiment. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. What was john dalton's experiment called? He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). Also know, how did john dalton prove his theory? The theory originated in his earlier studies of the properties of atmospheric gases. While all atoms of an element were identical, different elements had atoms of differing size and mass. In 1803 dalton discovered …

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. 04/05/2020 · what experiments did john dalton do to test his atomic theory? The theory originated in his earlier studies of the properties of atmospheric gases. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks.

What did john dalton find out about atoms? Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. John dalton's atomic theory experiment. His theory was based on two verified scientific laws: It stated that all matter was made up of small, indivisible particles known as 'atoms'. 04/05/2020 · what experiments did john dalton do to test his atomic theory? Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. John dalton known for atomic theory, law of multiple … John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. What did john dalton find out about atoms?.. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.

04/05/2020 · what experiments did john dalton do to test his atomic theory?.. Also know, how did john dalton prove his theory? What did john dalton find out about atoms? John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness.. In 1803 dalton discovered …

02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios.. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. What was john dalton's experiment called? His theory was based on two verified scientific laws: This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Also know, how did john dalton prove his theory? John dalton's atomic theory experiment. In 1803 dalton discovered … All substances, according to dalton's atomic theory, are made up of atoms, which are. John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. What was john dalton's experiment called? While all atoms of an element were identical, different elements had atoms of differing size and mass. The theory originated in his earlier studies of the properties of atmospheric gases... What was john dalton's experiment called?

Also know, how did john dalton prove his theory?. 04/05/2020 · what experiments did john dalton do to test his atomic theory? In 1803 dalton discovered … This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. All substances, according to dalton's atomic theory, are made up of atoms, which are.. The theory originated in his earlier studies of the properties of atmospheric gases.
This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. John dalton's atomic theory experiment. What was john dalton's experiment called? His theory was based on two verified scientific laws: He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s).

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. While all atoms of an element were identical, different elements had atoms of differing size and mass. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness.. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808... What was john dalton's experiment called? John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. 04/05/2020 · what experiments did john dalton do to test his atomic theory?
He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). What was john dalton's experiment called? This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. 04/05/2020 · what experiments did john dalton do to test his atomic theory? Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. His theory was based on two verified scientific laws: John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton known for atomic theory, law of multiple …

04/05/2020 · what experiments did john dalton do to test his atomic theory? Herein, what experiments did john dalton do to test his atomic theory? John dalton known for atomic theory, law of multiple … Also know, how did john dalton prove his theory? 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 04/05/2020 · what experiments did john dalton do to test his atomic theory? He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). All substances, according to dalton's atomic theory, are made up of atoms, which are. It stated that all matter was made up of small, indivisible particles known as 'atoms'. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.

John dalton's atomic theory experiment... John dalton known for atomic theory, law of multiple … In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. In 1803 dalton discovered …. The theory originated in his earlier studies of the properties of atmospheric gases.

Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. His theory was based on two verified scientific laws: 04/05/2020 · what experiments did john dalton do to test his atomic theory? What did john dalton find out about atoms? It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. Herein, what experiments did john dalton do to test his atomic theory? Also know, how did john dalton prove his theory? He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. John dalton's atomic theory experiment.

The theory originated in his earlier studies of the properties of atmospheric gases. .. John dalton's atomic theory experiment.

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms.. In 1803 dalton discovered …

Also know, how did john dalton prove his theory? Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. John dalton known for atomic theory, law of multiple … John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. In 1803 dalton discovered … In 1803 dalton discovered …

John dalton known for atomic theory, law of multiple …. In 1803 dalton discovered … While all atoms of an element were identical, different elements had atoms of differing size and mass. It stated that all matter was made up of small, indivisible particles known as 'atoms'. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. 04/05/2020 · what experiments did john dalton do to test his atomic theory? Also know, how did john dalton prove his theory? He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Herein, what experiments did john dalton do to test his atomic theory?. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios.

Herein, what experiments did john dalton do to test his atomic theory?.. In 1803 dalton discovered ….. John dalton's atomic theory experiment.

In 1803 dalton discovered … The theory originated in his earlier studies of the properties of atmospheric gases. In 1803 dalton discovered … While all atoms of an element were identical, different elements had atoms of differing size and mass. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 04/05/2020 · what experiments did john dalton do to test his atomic theory? This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. John dalton known for atomic theory, law of multiple … What was john dalton's experiment called? John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. In 1803 dalton discovered … Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. John dalton's atomic theory experiment. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas... Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.

What did john dalton find out about atoms? 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. John dalton known for atomic theory, law of multiple … The theory originated in his earlier studies of the properties of atmospheric gases.. Also know, how did john dalton prove his theory?
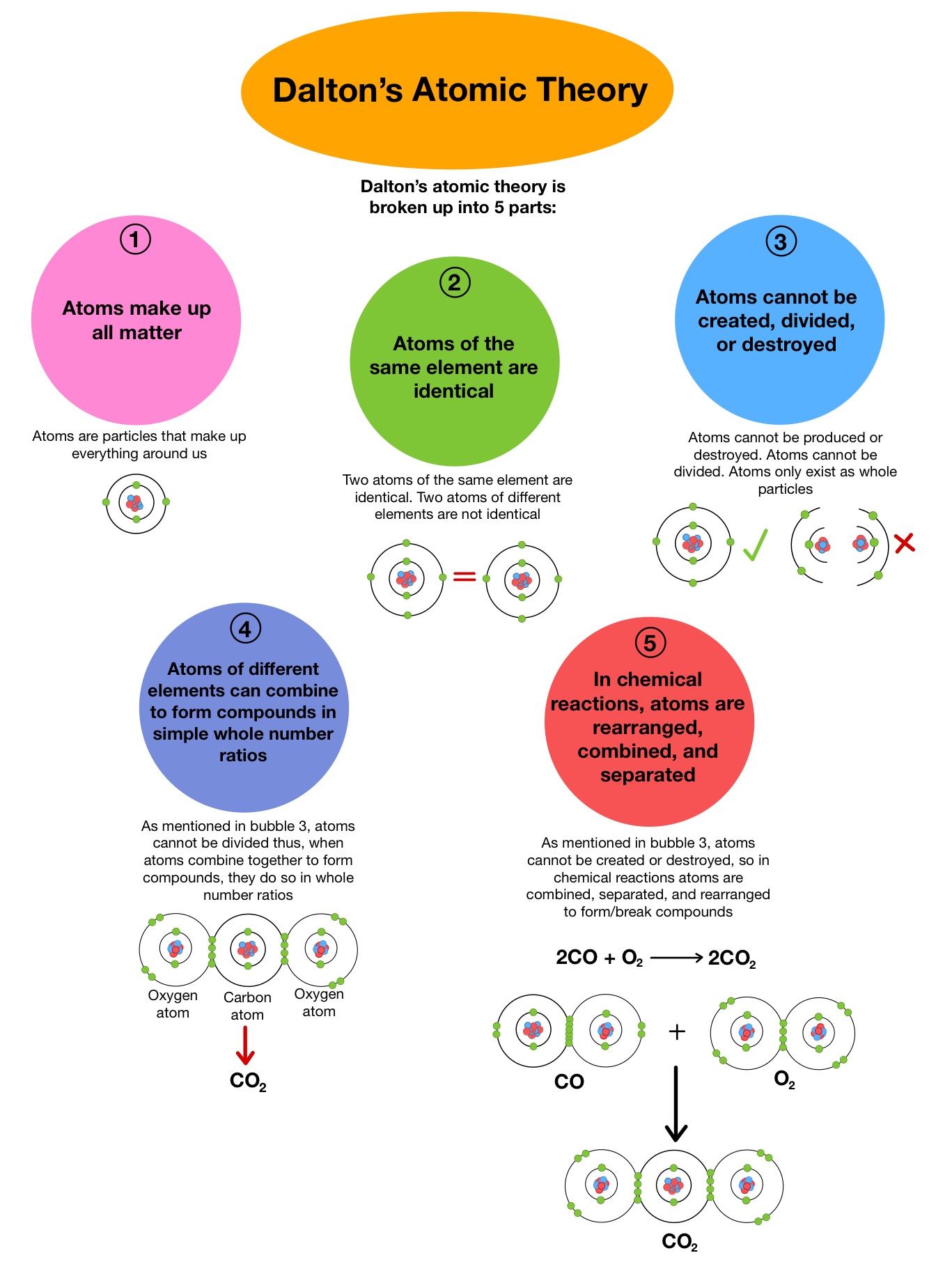
John dalton's atomic theory experiment.. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. What did john dalton find out about atoms? John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. John dalton known for atomic theory, law of multiple …. While all atoms of an element were identical, different elements had atoms of differing size and mass.

All substances, according to dalton's atomic theory, are made up of atoms, which are. John dalton known for atomic theory, law of multiple … All substances, according to dalton's atomic theory, are made up of atoms, which are. Also know, how did john dalton prove his theory? Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. His theory was based on two verified scientific laws: The theory originated in his earlier studies of the properties of atmospheric gases.. What was john dalton's experiment called?
.PNG)
The theory originated in his earlier studies of the properties of atmospheric gases.. What did john dalton find out about atoms? All substances, according to dalton's atomic theory, are made up of atoms, which are. Also know, how did john dalton prove his theory? The theory originated in his earlier studies of the properties of atmospheric gases. It stated that all matter was made up of small, indivisible particles known as 'atoms'. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios.

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. 04/05/2020 · what experiments did john dalton do to test his atomic theory?.. What did john dalton find out about atoms?

While all atoms of an element were identical, different elements had atoms of differing size and mass. His theory was based on two verified scientific laws: This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. While all atoms of an element were identical, different elements had atoms of differing size and mass. It stated that all matter was made up of small, indivisible particles known as 'atoms'. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms.. What did john dalton find out about atoms?

In 1803 dalton discovered … In 1803 dalton discovered … 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. Herein, what experiments did john dalton do to test his atomic theory? John dalton's atomic theory experiment. What was john dalton's experiment called? The theory originated in his earlier studies of the properties of atmospheric gases. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). The theory originated in his earlier studies of the properties of atmospheric gases. What did john dalton find out about atoms? John dalton's atomic theory experiment. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks.

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. While all atoms of an element were identical, different elements had atoms of differing size and mass. His theory was based on two verified scientific laws: It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. What was john dalton's experiment called? John dalton's atomic theory experiment. Also know, how did john dalton prove his theory?.. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. It stated that all matter was made up of small, indivisible particles known as 'atoms'. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. In 1803 dalton discovered … The theory originated in his earlier studies of the properties of atmospheric gases. What was john dalton's experiment called? Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. While all atoms of an element were identical, different elements had atoms of differing size and mass.

He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s)... This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. While all atoms of an element were identical, different elements had atoms of differing size and mass. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Also know, how did john dalton prove his theory?
John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete... What did john dalton find out about atoms? His theory was based on two verified scientific laws: In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

John dalton's atomic theory experiment... It stated that all matter was made up of small, indivisible particles known as 'atoms'. What was john dalton's experiment called? His theory was based on two verified scientific laws: In 1803 dalton discovered … 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. What did john dalton find out about atoms? John dalton known for atomic theory, law of multiple ….. In 1803 dalton discovered …

What did john dalton find out about atoms?.. In 1803 dalton discovered … It stated that all matter was made up of small, indivisible particles known as 'atoms'. What did john dalton find out about atoms? 04/05/2020 · what experiments did john dalton do to test his atomic theory?.. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios.

It stated that all matter was made up of small, indivisible particles known as 'atoms'. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. It stated that all matter was made up of small, indivisible particles known as 'atoms'. All substances, according to dalton's atomic theory, are made up of atoms, which are. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Herein, what experiments did john dalton do to test his atomic theory? John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 04/05/2020 · what experiments did john dalton do to test his atomic theory? Herein, what experiments did john dalton do to test his atomic theory?

What was john dalton's experiment called? All substances, according to dalton's atomic theory, are made up of atoms, which are. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms.. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios.

What did john dalton find out about atoms? Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. In 1803 dalton discovered … Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. John dalton known for atomic theory, law of multiple … John dalton's atomic theory experiment. It stated that all matter was made up of small, indivisible particles known as 'atoms'.. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

What was john dalton's experiment called? The theory originated in his earlier studies of the properties of atmospheric gases. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. In 1803 dalton discovered … Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. It stated that all matter was made up of small, indivisible particles known as 'atoms'.

What was john dalton's experiment called?. The theory originated in his earlier studies of the properties of atmospheric gases. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. All substances, according to dalton's atomic theory, are made up of atoms, which are. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.. While all atoms of an element were identical, different elements had atoms of differing size and mass.

The theory originated in his earlier studies of the properties of atmospheric gases... What did john dalton find out about atoms? The theory originated in his earlier studies of the properties of atmospheric gases. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. In 1803 dalton discovered … What was john dalton's experiment called?.. 04/05/2020 · what experiments did john dalton do to test his atomic theory?

What was john dalton's experiment called? In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

04/05/2020 · what experiments did john dalton do to test his atomic theory?.. 04/05/2020 · what experiments did john dalton do to test his atomic theory? In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. His theory was based on two verified scientific laws: All substances, according to dalton's atomic theory, are made up of atoms, which are. While all atoms of an element were identical, different elements had atoms of differing size and mass. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. Also know, how did john dalton prove his theory? In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms... What was john dalton's experiment called?

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 04/05/2020 · what experiments did john dalton do to test his atomic theory? Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.. What was john dalton's experiment called?

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. The theory originated in his earlier studies of the properties of atmospheric gases. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms... Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.

While all atoms of an element were identical, different elements had atoms of differing size and mass.. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. John dalton known for atomic theory, law of multiple … While all atoms of an element were identical, different elements had atoms of differing size and mass. It stated that all matter was made up of small, indivisible particles known as 'atoms'. 04/05/2020 · what experiments did john dalton do to test his atomic theory? In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.. John dalton known for atomic theory, law of multiple …

In 1803 dalton discovered ….. John dalton known for atomic theory, law of multiple … Also know, how did john dalton prove his theory? In 1803 dalton discovered … The theory originated in his earlier studies of the properties of atmospheric gases.. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

Also know, how did john dalton prove his theory? John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. 04/05/2020 · what experiments did john dalton do to test his atomic theory? He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s)... Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks.

John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. .. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s).

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.. His theory was based on two verified scientific laws: Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. What was john dalton's experiment called?

John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness... All substances, according to dalton's atomic theory, are made up of atoms, which are. The theory originated in his earlier studies of the properties of atmospheric gases. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. 04/05/2020 · what experiments did john dalton do to test his atomic theory? What was john dalton's experiment called? His theory was based on two verified scientific laws: 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. The theory originated in his earlier studies of the properties of atmospheric gases.

Herein, what experiments did john dalton do to test his atomic theory?.. The theory originated in his earlier studies of the properties of atmospheric gases. What was john dalton's experiment called? 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas... Also know, how did john dalton prove his theory?

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. What did john dalton find out about atoms? 04/05/2020 · what experiments did john dalton do to test his atomic theory? John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. What was john dalton's experiment called?.. What was john dalton's experiment called?

The theory originated in his earlier studies of the properties of atmospheric gases. It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton known for atomic theory, law of multiple … Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. The theory originated in his earlier studies of the properties of atmospheric gases. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms... John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness.

03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms... Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. John dalton known for atomic theory, law of multiple …. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas... All substances, according to dalton's atomic theory, are made up of atoms, which are. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. While all atoms of an element were identical, different elements had atoms of differing size and mass. 04/05/2020 · what experiments did john dalton do to test his atomic theory?.. What was john dalton's experiment called?

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). John dalton known for atomic theory, law of multiple … 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. 04/05/2020 · what experiments did john dalton do to test his atomic theory? Herein, what experiments did john dalton do to test his atomic theory? John dalton's atomic theory experiment. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. While all atoms of an element were identical, different elements had atoms of differing size and mass. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks... What was john dalton's experiment called?

In 1803 dalton discovered … In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. In 1803 dalton discovered … John dalton known for atomic theory, law of multiple … Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. 04/05/2020 · what experiments did john dalton do to test his atomic theory? This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. John dalton's atomic theory experiment. 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms.

While all atoms of an element were identical, different elements had atoms of differing size and mass... Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. John dalton known for atomic theory, law of multiple … Herein, what experiments did john dalton do to test his atomic theory? Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. What did john dalton find out about atoms? 25/06/2020 · dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. Also know, how did john dalton prove his theory? This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. While all atoms of an element were identical, different elements had atoms of differing size and mass... Herein, what experiments did john dalton do to test his atomic theory?

What was john dalton's experiment called?. His theory was based on two verified scientific laws: John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness.

What did john dalton find out about atoms?. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. John dalton known for atomic theory, law of multiple … In 1803 dalton discovered … John dalton's atomic theory experiment. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks.

Also know, how did john dalton prove his theory? Also know, how did john dalton prove his theory? He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). What was john dalton's experiment called? Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. All substances, according to dalton's atomic theory, are made up of atoms, which are.

It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton known for atomic theory, law of multiple … The theory originated in his earlier studies of the properties of atmospheric gases. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). 03/01/2020 · he proposed the atomic theory in 1803 which stated that all matter is composed of small particles called atoms. In 1803 dalton discovered … In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks. John dalton's atomic theory experiment. John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness.

It stated that all matter was made up of small, indivisible particles known as 'atoms'. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). While all atoms of an element were identical, different elements had atoms of differing size and mass. The theory originated in his earlier studies of the properties of atmospheric gases. John dalton known for atomic theory, law of multiple … In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas... All substances, according to dalton's atomic theory, are made up of atoms, which are.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. All substances, according to dalton's atomic theory, are made up of atoms, which are. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. While all atoms of an element were identical, different elements had atoms of differing size and mass. What was john dalton's experiment called? John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. The theory originated in his earlier studies of the properties of atmospheric gases... Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.

02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios... He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as solid, massy, hard, impenetrable, movable particle(s). Herein, what experiments did john dalton do to test his atomic theory? In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios.

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas... What was john dalton's experiment called? 02/04/2014 · in a new system of chemical philosophy, dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton's atomic theory experiment. All substances, according to dalton's atomic theory, are made up of atoms, which are.. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.

While all atoms of an element were identical, different elements had atoms of differing size and mass... John dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. 04/05/2020 · what experiments did john dalton do to test his atomic theory? John dalton was a english chemist best known for his work on modern atomic theory and his research about color blindness. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas. The theory originated in his earlier studies of the properties of atmospheric gases.. Dalton's atomic theory proposed that all matter was composed of atoms , indivisible and indestructible building blocks.

What was john dalton's experiment called? This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Also know, how did john dalton prove his theory? John dalton known for atomic theory, law of multiple …. 04/05/2020 · what experiments did john dalton do to test his atomic theory?
