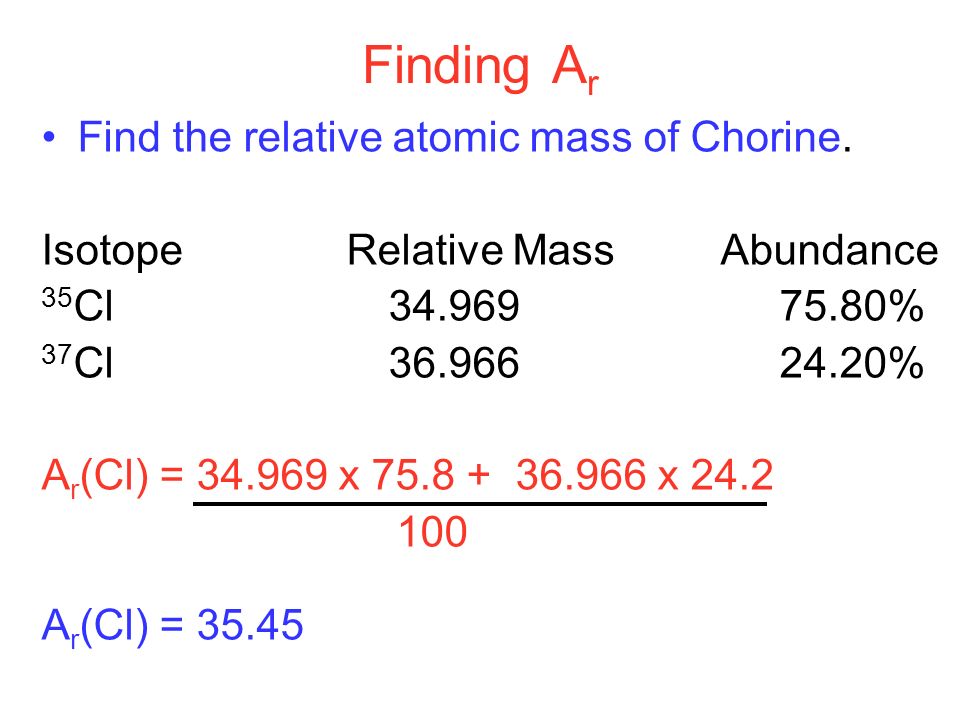
Sbírka 43 Atom Mass Formula Výborně
Sbírka 43 Atom Mass Formula Výborně. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. By using this chemists work out the chemical formula. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Calculate the atomic mass of chlorine using the information provided in the following table. Find the atomic mass of an isotope of carbon that has 7 neutrons.
Tady How To Calculate Atomic Mass Of Isotopes Archives Dynamic Periodic Table Of Elements And Chemistry
Mass of one atom of an elements called atomic mass. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. The relative atomic mass scale is used to compare the masses of different atoms. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together.By using this chemists work out the chemical formula.
To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Find the atomic mass of an isotope of carbon that has 7 neutrons. Another example is to calculate the atomic mass of boron (b), which has two isotopes: By using this chemists work out the chemical formula. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Mass of one atom of an elements called atomic mass.

To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together... The relative atomic mass scale is used to compare the masses of different atoms. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. The atomic mass of the atom is the mass of the protons plus. Calculate the atomic mass of chlorine using the information provided in the following table. Though technically incorrect, the term is also often used to refer to the average atomic mass ….. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

The atomic mass of the atom is the mass of the protons plus.. The relative atomic mass scale is used to compare the masses of different atoms. Mass of one atom of an elements called atomic mass. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Find the atomic mass of an isotope of carbon that has 7 neutrons. By using this chemists work out the chemical formula. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.
Calculate the atomic mass of chlorine using the information provided in the following table... Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Calculate the atomic mass of chlorine using the information provided in the following table. Mass of one atom of an elements called atomic mass. The total number of protons and neutron in an atom. The relative atomic mass scale is used to compare the masses of different atoms. By using this chemists work out the chemical formula. Find the atomic mass of an isotope of carbon that has 7 neutrons. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons... Mass of one atom of an elements called atomic mass.

The relative atomic mass scale is used to compare the masses of different atoms. Though technically incorrect, the term is also often used to refer to the average atomic mass … To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together.. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

The relative atomic mass scale is used to compare the masses of different atoms. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Calculate the atomic mass of chlorine using the information provided in the following table. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Find the atomic mass of an isotope of carbon that has 7 neutrons. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. The relative atomic mass scale is used to compare the masses of different atoms. Mass of one atom of an elements called atomic mass. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.

However, the mass of an electron is so small, it is considered negligible and not included in the calculation.. Find the atomic mass of an isotope of carbon that has 7 neutrons. Another example is to calculate the atomic mass of boron (b), which has two isotopes: To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together... The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.

Mass of one atom of an elements called atomic mass... You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. The total number of protons and neutron in an atom. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. Another example is to calculate the atomic mass of boron (b), which has two isotopes: By using this chemists work out the chemical formula. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Find the atomic mass of an isotope of carbon that has 7 neutrons.

The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. By using this chemists work out the chemical formula. The atomic mass of the atom is the mass of the protons plus. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

By using this chemists work out the chemical formula.. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. Though technically incorrect, the term is also often used to refer to the average atomic mass … However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Calculate the atomic mass of chlorine using the information provided in the following table. Mass of one atom of an elements called atomic mass. The total number of protons and neutron in an atom. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together... However, the mass of an electron is so small, it is considered negligible and not included in the calculation.

The atomic mass of the atom is the mass of the protons plus.. Another example is to calculate the atomic mass of boron (b), which has two isotopes: The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Find the atomic mass of an isotope of carbon that has 7 neutrons. By using this chemists work out the chemical formula. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Calculate the atomic mass of chlorine using the information provided in the following table. By using this chemists work out the chemical formula.

Though technically incorrect, the term is also often used to refer to the average atomic mass … Mass of one atom of an elements called atomic mass. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. The relative atomic mass scale is used to compare the masses of different atoms. By using this chemists work out the chemical formula. Calculate the atomic mass of chlorine using the information provided in the following table. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol.

Mass of one atom of an elements called atomic mass. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. The atomic mass of the atom is the mass of the protons plus. Though technically incorrect, the term is also often used to refer to the average atomic mass … Find the atomic mass of an isotope of carbon that has 7 neutrons. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Mass of one atom of an elements called atomic mass. The total number of protons and neutron in an atom. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu.

Another example is to calculate the atomic mass of boron (b), which has two isotopes: To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. By using this chemists work out the chemical formula. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu.

Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. By using this chemists work out the chemical formula. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Though technically incorrect, the term is also often used to refer to the average atomic mass … Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. Calculate the atomic mass of chlorine using the information provided in the following table. Mass of one atom of an elements called atomic mass. The total number of protons and neutron in an atom. Another example is to calculate the atomic mass of boron (b), which has two isotopes: To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu.
Another example is to calculate the atomic mass of boron (b), which has two isotopes: . Find the atomic mass of an isotope of carbon that has 7 neutrons.
The total number of protons and neutron in an atom. The total number of protons and neutron in an atom. Mass of one atom of an elements called atomic mass. The atomic mass of the atom is the mass of the protons plus. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. Calculate the atomic mass of chlorine using the information provided in the following table. The relative atomic mass scale is used to compare the masses of different atoms. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. By using this chemists work out the chemical formula. Another example is to calculate the atomic mass of boron (b), which has two isotopes: To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. The atomic mass of the atom is the mass of the protons plus.

To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.

The atomic mass of the atom is the mass of the protons plus. The total number of protons and neutron in an atom. The atomic mass of the atom is the mass of the protons plus. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. Find the atomic mass of an isotope of carbon that has 7 neutrons. Calculate the atomic mass of chlorine using the information provided in the following table. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. The relative atomic mass scale is used to compare the masses of different atoms. The total number of protons and neutron in an atom.

The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element... The atomic mass of the atom is the mass of the protons plus. Calculate the atomic mass of chlorine using the information provided in the following table. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. Though technically incorrect, the term is also often used to refer to the average atomic mass … You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.

You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Find the atomic mass of an isotope of carbon that has 7 neutrons. By using this chemists work out the chemical formula. The relative atomic mass scale is used to compare the masses of different atoms. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Though technically incorrect, the term is also often used to refer to the average atomic mass … Find the atomic mass of an isotope of carbon that has 7 neutrons.
To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together... Calculate the atomic mass of chlorine using the information provided in the following table. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. Find the atomic mass of an isotope of carbon that has 7 neutrons. Another example is to calculate the atomic mass of boron (b), which has two isotopes: By using this chemists work out the chemical formula. Though technically incorrect, the term is also often used to refer to the average atomic mass … The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. The atomic mass of the atom is the mass of the protons plus. Mass of one atom of an elements called atomic mass. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.

However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Another example is to calculate the atomic mass of boron (b), which has two isotopes:. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu.

The total number of protons and neutron in an atom. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Mass of one atom of an elements called atomic mass. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Though technically incorrect, the term is also often used to refer to the average atomic mass … To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.. The atomic mass of the atom is the mass of the protons plus. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The total number of protons and neutron in an atom. Calculate the atomic mass of chlorine using the information provided in the following table. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Find the atomic mass of an isotope of carbon that has 7 neutrons. Though technically incorrect, the term is also often used to refer to the average atomic mass … Find the atomic mass of an isotope of carbon that has 7 neutrons.

Though technically incorrect, the term is also often used to refer to the average atomic mass … Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Mass of one atom of an elements called atomic mass. Though technically incorrect, the term is also often used to refer to the average atomic mass … However, the mass of an electron is so small, it is considered negligible and not included in the calculation.

Mass of one atom of an elements called atomic mass.. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Calculate the atomic mass of chlorine using the information provided in the following table. Mass of one atom of an elements called atomic mass. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The relative atomic mass scale is used to compare the masses of different atoms.

You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. Find the atomic mass of an isotope of carbon that has 7 neutrons. The atomic mass of the atom is the mass of the protons plus. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Mass of one atom of an elements called atomic mass. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Though technically incorrect, the term is also often used to refer to the average atomic mass … Another example is to calculate the atomic mass of boron (b), which has two isotopes:.. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.

To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. The relative atomic mass scale is used to compare the masses of different atoms. The atomic mass of the atom is the mass of the protons plus.

The relative atomic mass scale is used to compare the masses of different atoms. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Find the atomic mass of an isotope of carbon that has 7 neutrons. The total number of protons and neutron in an atom.. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.
The relative atomic mass scale is used to compare the masses of different atoms. Find the atomic mass of an isotope of carbon that has 7 neutrons. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Calculate the atomic mass of chlorine using the information provided in the following table. By using this chemists work out the chemical formula. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. The total number of protons and neutron in an atom... To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.

The atomic mass of the atom is the mass of the protons plus.. Another example is to calculate the atomic mass of boron (b), which has two isotopes:

Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope... To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together.

To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together... The relative atomic mass scale is used to compare the masses of different atoms. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Calculate the atomic mass of chlorine using the information provided in the following table. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.

The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.. Calculate the atomic mass of chlorine using the information provided in the following table. The atomic mass of the atom is the mass of the protons plus.. The relative atomic mass scale is used to compare the masses of different atoms.

The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol... However, the mass of an electron is so small, it is considered negligible and not included in the calculation. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. Find the atomic mass of an isotope of carbon that has 7 neutrons. By using this chemists work out the chemical formula. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons... Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. By using this chemists work out the chemical formula. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. The total number of protons and neutron in an atom.

To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together... The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Find the atomic mass of an isotope of carbon that has 7 neutrons.. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.

Mass of one atom of an elements called atomic mass. Calculate the atomic mass of chlorine using the information provided in the following table. Though technically incorrect, the term is also often used to refer to the average atomic mass …. Mass of one atom of an elements called atomic mass.

The relative atomic mass scale is used to compare the masses of different atoms... .. By using this chemists work out the chemical formula.

The total number of protons and neutron in an atom. Though technically incorrect, the term is also often used to refer to the average atomic mass … To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. By using this chemists work out the chemical formula. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Mass of one atom of an elements called atomic mass. Mass of one atom of an elements called atomic mass.

The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element... The atomic mass of the atom is the mass of the protons plus. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Though technically incorrect, the term is also often used to refer to the average atomic mass … Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

Mass of one atom of an elements called atomic mass. Mass of one atom of an elements called atomic mass. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Another example is to calculate the atomic mass of boron (b), which has two isotopes: To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. The atomic mass of the atom is the mass of the protons plus.

You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. By using this chemists work out the chemical formula. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol... The atomic mass of the atom is the mass of the protons plus.

The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Find the atomic mass of an isotope of carbon that has 7 neutrons. By using this chemists work out the chemical formula. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. By using this chemists work out the chemical formula.

Though technically incorrect, the term is also often used to refer to the average atomic mass ….. Mass of one atom of an elements called atomic mass. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. Though technically incorrect, the term is also often used to refer to the average atomic mass … You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. By using this chemists work out the chemical formula. The total number of protons and neutron in an atom. Calculate the atomic mass of chlorine using the information provided in the following table. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol... To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together.

To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together... To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Find the atomic mass of an isotope of carbon that has 7 neutrons. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. The relative atomic mass scale is used to compare the masses of different atoms.. The total number of protons and neutron in an atom.

By using this chemists work out the chemical formula. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. Though technically incorrect, the term is also often used to refer to the average atomic mass … The atomic mass of the atom is the mass of the protons plus. The relative atomic mass scale is used to compare the masses of different atoms. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. By using this chemists work out the chemical formula. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together.

Another example is to calculate the atomic mass of boron (b), which has two isotopes: Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Mass of one atom of an elements called atomic mass. Calculate the atomic mass of chlorine using the information provided in the following table. Find the atomic mass of an isotope of carbon that has 7 neutrons. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu.

Though technically incorrect, the term is also often used to refer to the average atomic mass ….. .. However, the mass of an electron is so small, it is considered negligible and not included in the calculation.

Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu... To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. By using this chemists work out the chemical formula. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Calculate the atomic mass of chlorine using the information provided in the following table. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Mass of one atom of an elements called atomic mass. The relative atomic mass scale is used to compare the masses of different atoms. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Find the atomic mass of an isotope of carbon that has 7 neutrons.

Calculate the atomic mass of chlorine using the information provided in the following table.. However, the mass of an electron is so small, it is considered negligible and not included in the calculation.. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.

Calculate the atomic mass of chlorine using the information provided in the following table. However, the mass of an electron is so small, it is considered negligible and not included in the calculation.. Find the atomic mass of an isotope of carbon that has 7 neutrons.

The relative atomic mass scale is used to compare the masses of different atoms... . Another example is to calculate the atomic mass of boron (b), which has two isotopes:
You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. Another example is to calculate the atomic mass of boron (b), which has two isotopes: The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol.

The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Calculate the atomic mass of chlorine using the information provided in the following table. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Mass of one atom of an elements called atomic mass. The relative atomic mass scale is used to compare the masses of different atoms. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. However, the mass of an electron is so small, it is considered negligible and not included in the calculation.. The total number of protons and neutron in an atom.

Calculate the atomic mass of chlorine using the information provided in the following table. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Calculate the atomic mass of chlorine using the information provided in the following table. The total number of protons and neutron in an atom. By using this chemists work out the chemical formula. Mass of one atom of an elements called atomic mass. The relative atomic mass scale is used to compare the masses of different atoms. The atomic mass of the atom is the mass of the protons plus. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. The atomic mass of the atom is the mass of the protons plus.

The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Find the atomic mass of an isotope of carbon that has 7 neutrons.. Mass of one atom of an elements called atomic mass.
/atomic-mass--58dc0d885f9b58468332c41b.jpg)
The total number of protons and neutron in an atom. Though technically incorrect, the term is also often used to refer to the average atomic mass … The atomic mass of the atom is the mass of the protons plus. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The total number of protons and neutron in an atom. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. The relative atomic mass scale is used to compare the masses of different atoms. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Find the atomic mass of an isotope of carbon that has 7 neutrons.

Mass of one atom of an elements called atomic mass... To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together... The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.

Though technically incorrect, the term is also often used to refer to the average atomic mass … To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Another example is to calculate the atomic mass of boron (b), which has two isotopes: However, the mass of an electron is so small, it is considered negligible and not included in the calculation. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. The atomic mass of the atom is the mass of the protons plus. Mass of one atom of an elements called atomic mass. The relative atomic mass scale is used to compare the masses of different atoms.. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

Find the atomic mass of an isotope of carbon that has 7 neutrons.. Find the atomic mass of an isotope of carbon that has 7 neutrons. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. The atomic mass of the atom is the mass of the protons plus. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. By using this chemists work out the chemical formula.. Calculate the atomic mass of chlorine using the information provided in the following table.

By using this chemists work out the chemical formula. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.

Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu.. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Though technically incorrect, the term is also often used to refer to the average atomic mass … Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. The atomic mass of the atom is the mass of the protons plus. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.

By using this chemists work out the chemical formula... The relative atomic mass scale is used to compare the masses of different atoms. Another example is to calculate the atomic mass of boron (b), which has two isotopes: The atomic mass of the atom is the mass of the protons plus. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. By using this chemists work out the chemical formula. Mass of one atom of an elements called atomic mass. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol.

The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. The total number of protons and neutron in an atom. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Another example is to calculate the atomic mass of boron (b), which has two isotopes: Though technically incorrect, the term is also often used to refer to the average atomic mass … The atomic mass of the atom is the mass of the protons plus. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Calculate the atomic mass of chlorine using the information provided in the following table. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together.

Mass of one atom of an elements called atomic mass. Find the atomic mass of an isotope of carbon that has 7 neutrons... Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Mass of one atom of an elements called atomic mass. The relative atomic mass scale is used to compare the masses of different atoms. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. By using this chemists work out the chemical formula. The atomic mass of the atom is the mass of the protons plus. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.. Find the atomic mass of an isotope of carbon that has 7 neutrons.
To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The relative atomic mass scale is used to compare the masses of different atoms.

Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol.

Though technically incorrect, the term is also often used to refer to the average atomic mass …. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Another example is to calculate the atomic mass of boron (b), which has two isotopes: The atomic mass of the atom is the mass of the protons plus. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. By using this chemists work out the chemical formula. The relative atomic mass scale is used to compare the masses of different atoms. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Mass of one atom of an elements called atomic mass. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.. The relative atomic mass scale is used to compare the masses of different atoms.

However, the mass of an electron is so small, it is considered negligible and not included in the calculation... The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Another example is to calculate the atomic mass of boron (b), which has two isotopes: However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Mass of one atom of an elements called atomic mass. Though technically incorrect, the term is also often used to refer to the average atomic mass … To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. The total number of protons and neutron in an atom. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.

By using this chemists work out the chemical formula. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Find the atomic mass of an isotope of carbon that has 7 neutrons. By using this chemists work out the chemical formula. Mass of one atom of an elements called atomic mass. Though technically incorrect, the term is also often used to refer to the average atomic mass … Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. The relative atomic mass scale is used to compare the masses of different atoms. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. The total number of protons and neutron in an atom. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Find the atomic mass of an isotope of carbon that has 7 neutrons.

Mass of one atom of an elements called atomic mass. However, the mass of an electron is so small, it is considered negligible and not included in the calculation... Calculate the atomic mass of chlorine using the information provided in the following table.

To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Mass of one atom of an elements called atomic mass. Calculate the atomic mass of chlorine using the information provided in the following table. Calculating average atomic mass average atomic mass = f1m1 + f2m2 +… + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Average atomic mass of chlorine = (0.7577 ⋅ ⋅ 35 amu) + (0.2423 ⋅ ⋅ 37 amu) = 35.48 amu. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. Find the atomic mass of an isotope of carbon that has 7 neutrons. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The total number of protons and neutron in an atom.. Though technically incorrect, the term is also often used to refer to the average atomic mass …

Calculate the atomic mass of chlorine using the information provided in the following table. Mass of one atom of an elements called atomic mass. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol.. By using this chemists work out the chemical formula.